Understanding what cannot be seen. Dealing with the unknown. Breaking down concepts into forms and reassembling these forms into biological machines. Uncovering the latent universe of enzymes involves all these stages, and conquering their mysteries requires a lot of science and creativity. Enzymes (which are mostly proteins) are invisible to the naked eye and known to be catalysts: molecules that can accelerate chemical reactions that are essential for the function of complex organic systems such as the human body.
But the body represents only a small sample of enzymatic activities. They are everywhere and used in a multitude of processes from vaccine production to neutralizing lactose in milk for those who cannot tolerate this sugar, from producing biofuels to developing renewable chemicals.
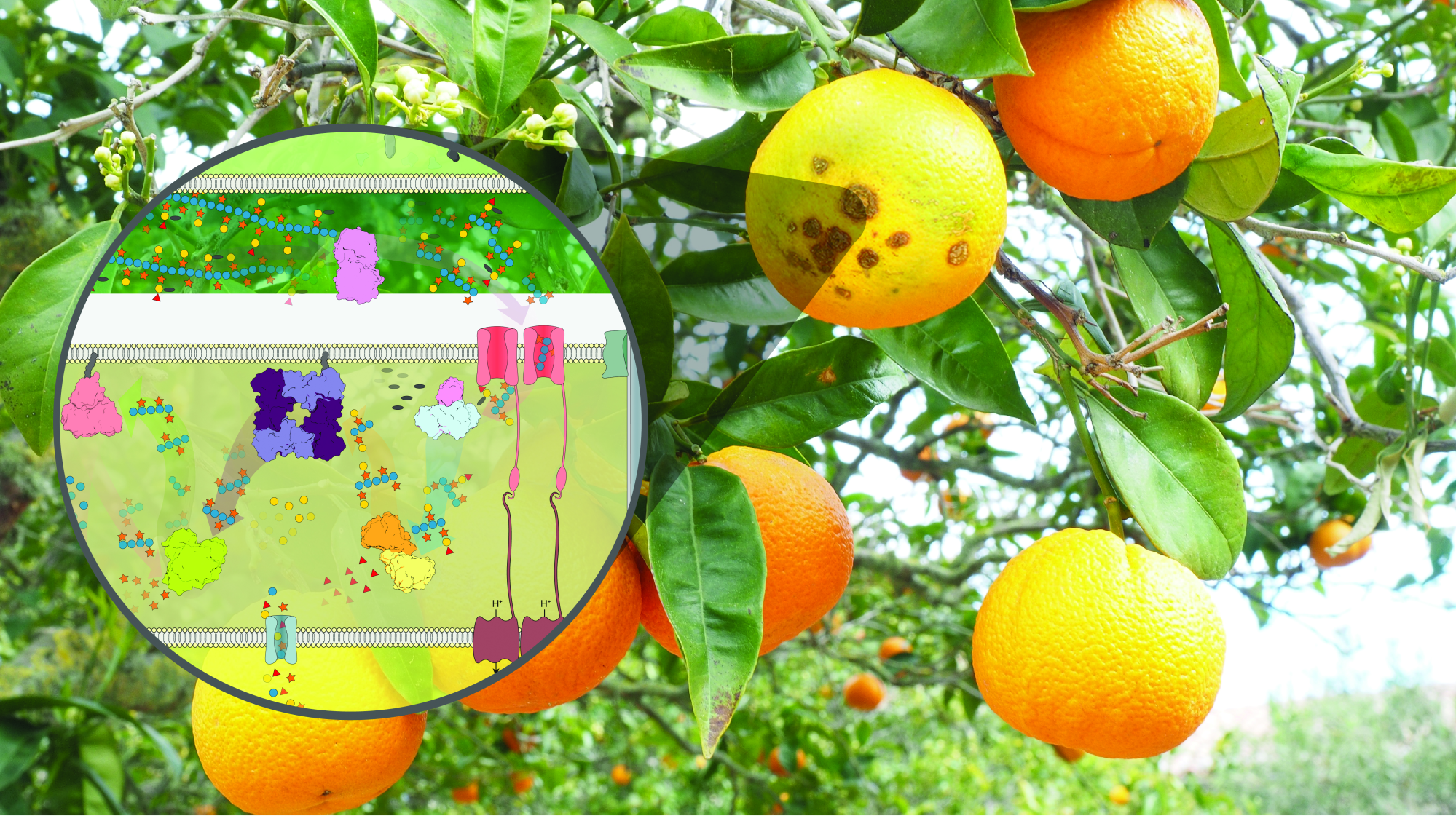
In bioproduct manufacturing, for example, enzymes can be applied to wastes resulting from agricultural processes in large quantities such as residue from orange juice and sugar production, straw, and other materials that until recently were considered low-value-added byproducts. This process known as cleavage makes it possible to obtain acids, solvents, polymers, and advanced biofuels, always using renewable sources and biological approaches.
Enzymes are consequently tools that can completely replace petroleum-based reagents that are currently used to transform molecules into high value-added products. The shift from an oil matrix to biological matrix is equally effective, but with dramatically less environmental impact. The challenge lies in finding the most appropriate enzymes for each desired application.
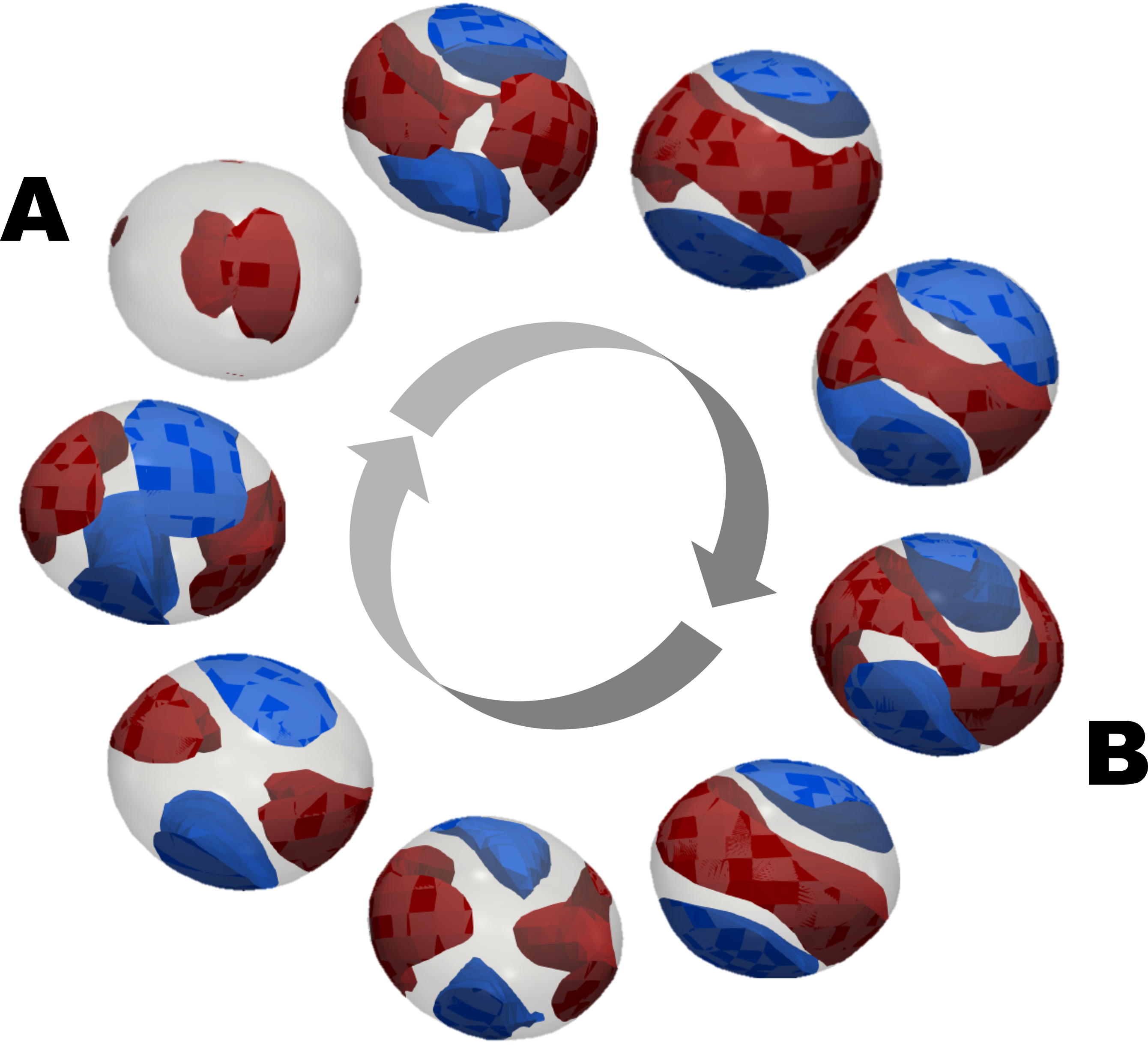
Identifying these molecules and applying them to the best tasks mobilizes large teams and state-of-the-art instrumentation based on synchrotron light, atomic tools, and bioinformatics. Between 2020 and the first half of 2021, CNPEM researchers identified a group of hitherto unknown enzymes and clarified the mechanism of action for another protein. In the study published in 2020 in Nature Chemical Biology, scientists revealed how a new family of enzymes applied innovative mechanisms and strategies to break down plant polysaccharides and generate useful byproducts such as prebiotics and biofuels.
In 2021, this time in Nature Communications, a new study shattered paradigms with the revelation that enzymes which act on hemicellulose can cleave glycoside connections (converting substrates into products) by two catalytic routes, contrary to prior expectations. In scientific terms, a catalytic route or itinerary represents the chemical and structural changes that a substrate (such as polysaccharides and carbohydrates) undergoes due to the action of the enzyme until it is converted into a product. This set of modifications was considered unique for a given enzyme and its substrate, but the study described pathways that could modify the theoretical understanding.
SEEKING INSPIRATION FROM EVERYTHING AROUND US
Both projects have a common inspiration: nature. Fungi and bacteria are enzyme-producing machines. When a pathogen attacks a plant, for example, the physical barrier is broken by enzymes that disintegrate the plant cell wall and allow the host to enter. Despite the undesirable effects on plants, this is the type of strategy researchers are looking at.
“We observe nature and formulate hypotheses. If an enzyme can rupture the physical barrier in plants, it may be able to do the same with agro-industrial waste, accessing trapped sugars and making them available for fermentation and conversion into products,” says Mario Murakami, scientific director of the LNBR and lead author of the studies.
Absorbing this knowledge and reproducing it in the laboratory is another important front for the Laboratory. CNPEM develops biological platforms (microorganisms) “engineered” via genetic modifications that can be customized to produce enzymes made to order, with potential applications in different activities.
“Our goal is simple: to emulate and improve molecular strategies found in nature that have been shaped over hundreds of millions of years,” he explains.
In CNPEM, enzymes are subjected to crystallography techniques with synchrotron radiation using Sirius, for example, in order to visualize the three-dimensional structure. Other steps include changes in molecular organization, laboratory testing, and pilot plant scaling to determine the feasibility of applying processes in supercritical environments such as manufacturing.
DO ENZYMES WORK ALONE?
The saying “two heads are better than one” can be extrapolated to enzymes. In general terms, different enzymes are synergistically combined to carry out a given activity. For example, dismantling agro-industrial byproducts into fermentable sugars requires a veritable arsenal comprising dozens of enzymes, which we call enzymatic cocktails.
“These enzyme formulations are complex combinations that make it possible to extract components of agro-industrial waste that can be biologically transformed into products of interest to society.”
The CNPEM has experience with such cocktails; one was developed to produce cellulosic ethanol (also known as second-generation ethanol) and patented in 2019 by the National Institute of Industrial Property (INPI) via the Patent Cooperation Treaty (PCT). In this way, CNPEM controls all the stages involved in producing this enzymatic ordnance: engineering the biological platforms (microorganisms that produce enzymes), customizing the enzymes, and developing the cocktail.
These characteristics put Brazil in a prominent position in both scientific and technological terms. By owning the enzyme development and production chain, the country is more competitive, with manufacturers needing to import less and consequently reducing their carbon footprints by using enzymes made locally or even on site.